How to Prepare a PDE Report in 5 Steps
Step 1: Identification of Potential Hazards
All relevant clinical and non-clinical data are reviewed.
Step 2: Identification of “Critical Effects”
Safety concerns detailed in the Risk Management Plan of the final product are reviewed in order to detect other critical effects in humans.
Critical effects in humans from mechanistic studies and pharmacodynamics data are discussed in the report.
Step 3: Establishing a Point of Departure for PDE calculation
A No Observed Adverse Effect Level (“NOAEL”) corresponding to the highest dose tested at which no “critical” effect occurs should be determined for each critical effect identified.
- When several NOAELs are available,
=> the lowest value and the most sensitive animal are selected.
- In the absence of NOAEL,
=> a Lowest Observed Adverse Effect Level (“LOAEL”) can be used.
First-in-human-Phase 1 clinical studies are reviewed in order to identify a human NOEL.
NOEL in healthy volunteers is the highest exposure level at which there are no effects (adverse or non-adverse) and has preference to calculate a PDE over NOAEL in animals.
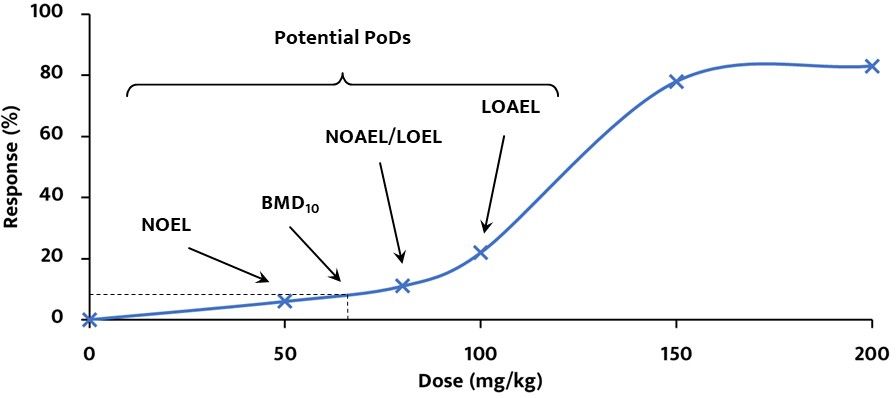
- NOEL: greatest concentration or amount of substance that causes no changes under defined conditions of exposure
- NOAEL: greatest concentration or amount of substance that causes no detectable adverse changes under defined conditions of exposure
- LOEL: lowest concentration or amount of substance that causes any effect under defined conditions of exposure
- LOAEL: lowest concentration or amount of substance that causes an adverse effect under defined conditions of exposure
- BMD10: statistically calculated dose that produces a defined response (in this case, 10 %) of an adverse effect compare to background
Step 4 – Selection of Correction Factors
Several adjustments factors are used to account for various uncertainties.
The specific pharmacokinetics of each drug product are taken into consideration to adjust the bioavailability factor.
Step 5 − PDE Calculation
All relevant and recent articles published regarding methods of calculating PDE, in addition to the EMA guidelines, are considered; for example:
- Lovsin Barle et al. PDE for topical ocular drugs (Pharm Dev Technol. 2018;23(3):225-230)
- Lovsin Barle et al. PDE concept for contaminants of intravitreal (IVT) drugs (Regul Toxicol Pharmacol. 2019;101:29-34)
For a given API:
- All parameters and sensitive species are taken into account to calculate different PDE values
- The choice of the selected PDE is explained
- The critical effects observed in non-clinical toxicity studies are studied in order to evaluate their relevance for the human or the target animal
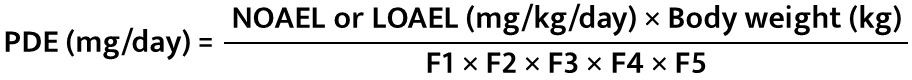
ToxBy.Design can develop PDE monograph for Active Substances or Chemicals and provide specific reports for these categories.
You can check Tox by Design methodology for PDE Monograph, which are duly validated and signed by an European Registered Toxicologist expert.
In the event you are performing this exercise for innovative compounds GMP scale up manufacturing, please be noted that Tox by Design is duly accredited for the French Research Tax Credit CIR.
Feel free to contact us using the Quotation Request below to receive a quotation for PDE Monograph for your Active Pharmaceutical Ingredient, New Chemical or Biological Entity or any Chemicals substances deployed in your GMP manufacturing lines.